Study selection. Flow chart shows the selection process starting with... | Download Scientific Diagram

EU Medicines Agency on Twitter: "EMA has published the full assessment report for #COVID19vaccine AstraZeneca, together with the product information in all official EU languages: 👉 https://t.co/WAP1UBgJGe https://t.co/iu4V1lfTim" / Twitter
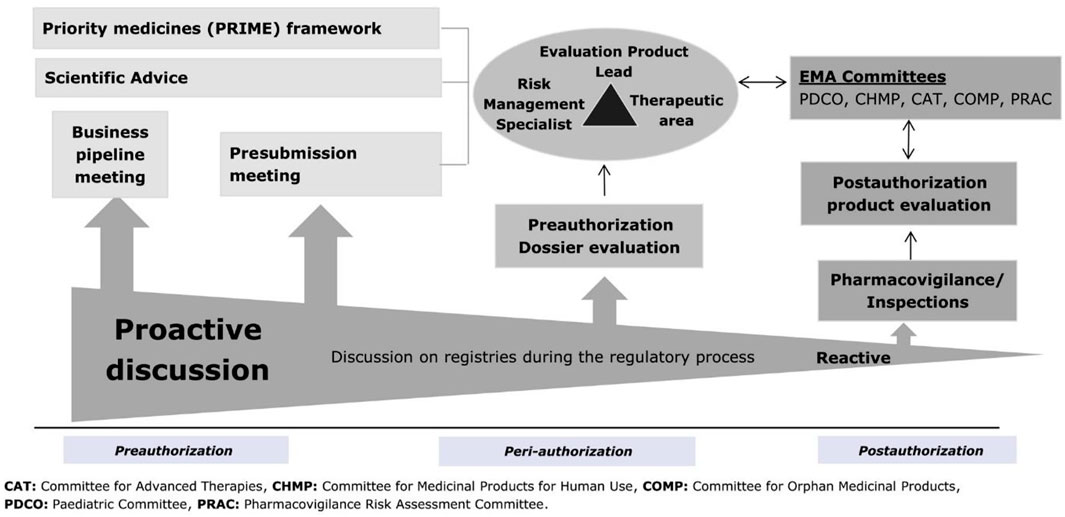
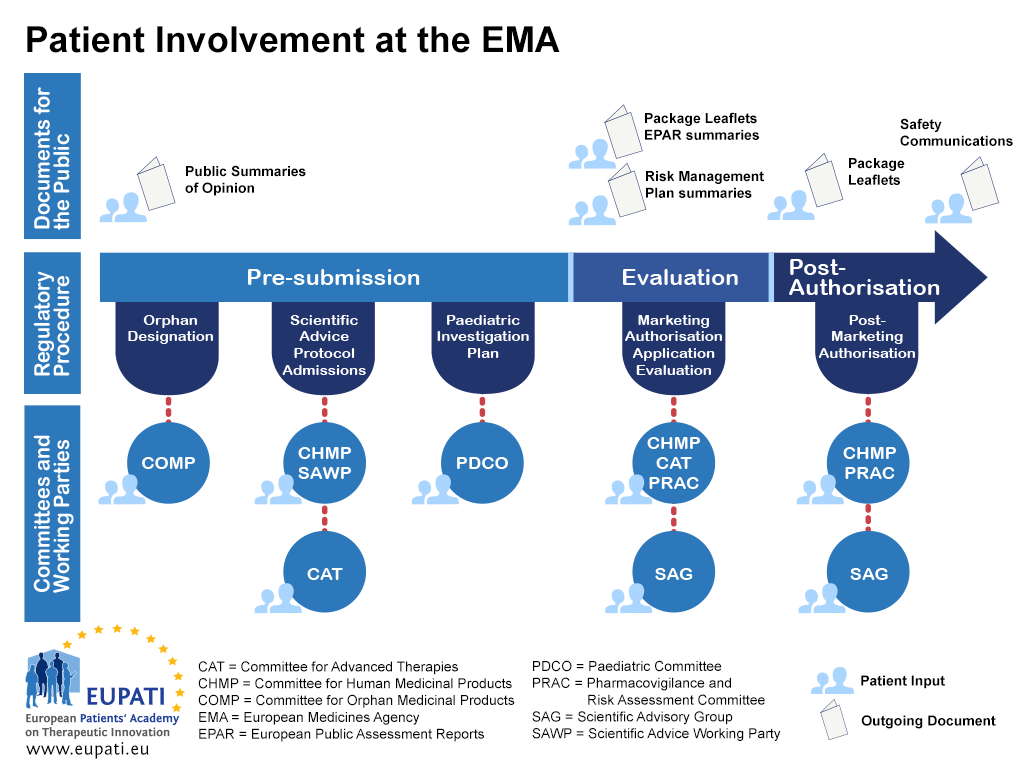
Frontiers | Contribution of patient registries to regulatory decision making on rare diseases medicinal products in Europe

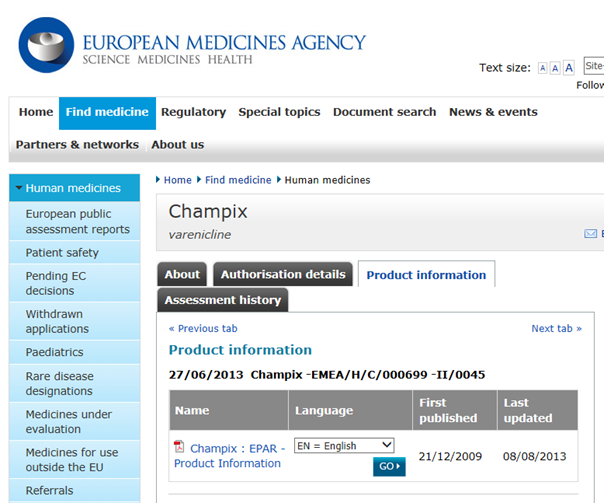
European Public Assessment Report (EPAR) summaries for the public: are they fit for purpose? A user-testing study | BMJ Open
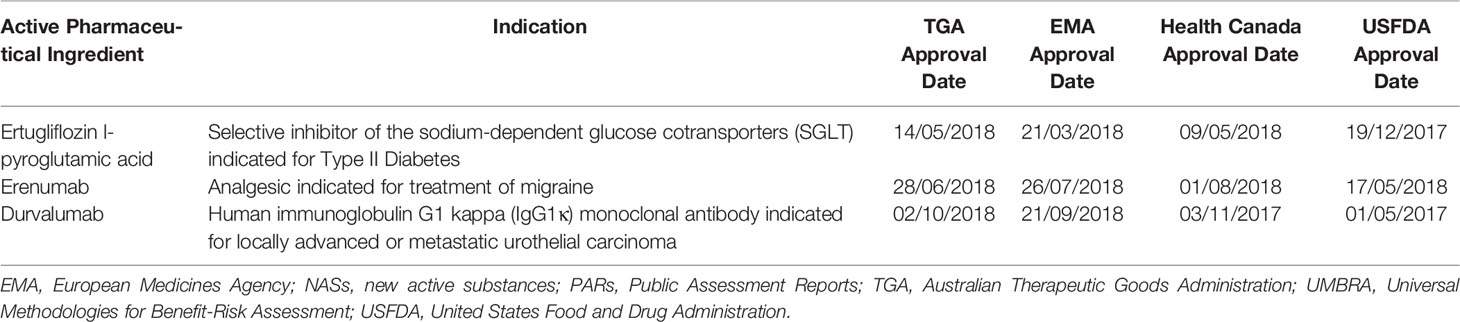
Improving the Contribution of Regulatory Assessment Reports to Health Technology Assessments—A Collaboration between the Europ

Assessment Report EMA/Pfizer-BioNTech Comirnaty, February 19, 2021,... | Download Scientific Diagram

Assessment Report EMA/Pfizer-BioNTech Comirnaty, February 19, 2021,... | Download Scientific Diagram
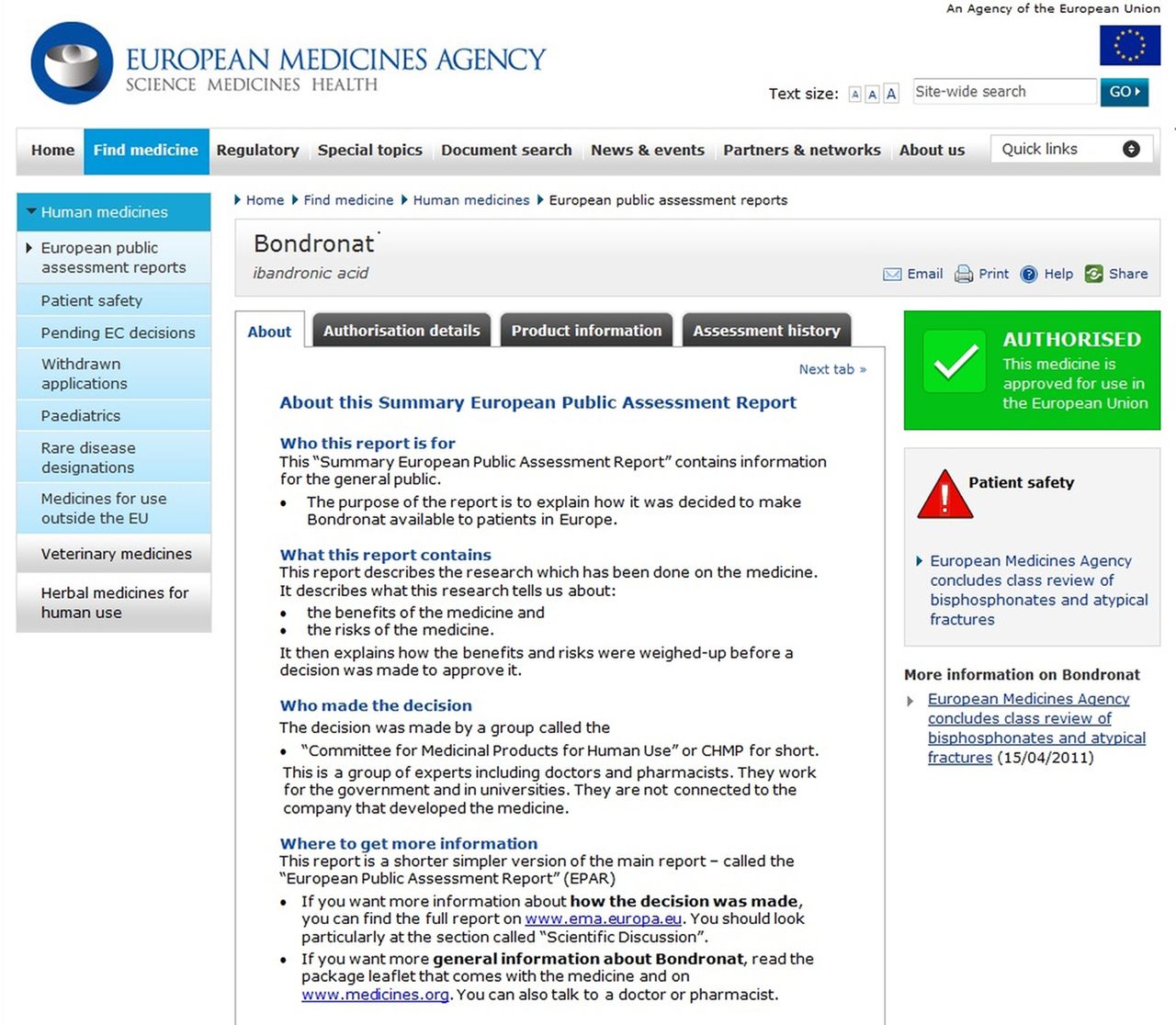
European Public Assessment Report (EPAR) summaries for the public: are they fit for purpose? A user-testing study | BMJ Open