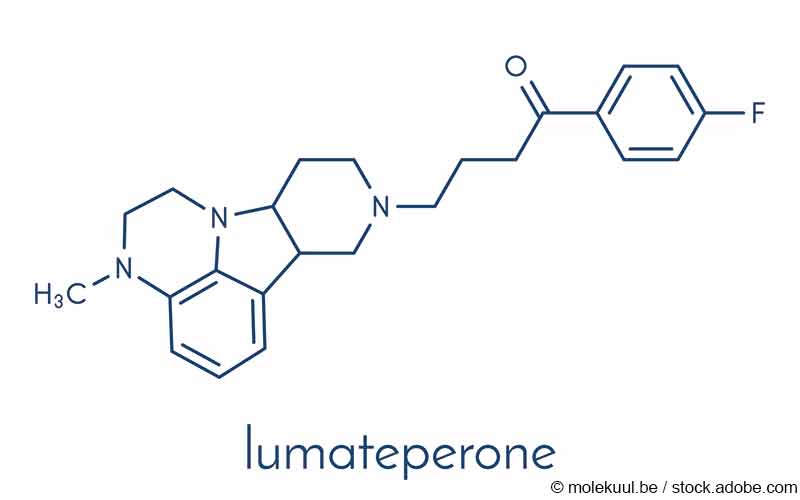
Lumateperone: antipsicotico con ridotti effetti avversi metabolici di risperidone – Centro Regionale FarmacoVigilanza Sardegna

PDF) Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. (2019) | Filippo Corponi | 33 Citations