AbbVie wins CHMP positive opinion to use Skyrizi as an active psoriatic arthritis treatment - Drug Discovery and Development

SIFO - Società Italiana di Farmacia Ospedaliera e dei servizi farmaceutici delle aziende sanitarie - CHMP: meeting highlights del 12-15 settembre 2022

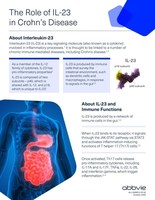
AbbVie Announces European Commission Approval of SKYRIZI® (risankizumab) for the Treatment of Moderate to Severe Active Crohn's Disease | AbbVie News Center

Twitter-এ Dividend Wave: "$ABBV Announces European Commission Approval of Skyrizi for the treatment of moderate to severe active Crohn's Disease (CD) • Third indication in EU • First specific IL-23 inhibitor for